Financing
The study is funded by the EU Horizon 2020 program
The COTTIS-trials
Despite the well-established benefit of endovascular treatment (EVT) for acute ischemic stroke due to large vessel occlusion (LVO), more than half of patients treated with EVT remain functionally dependent despite high reperfusion rates. Thus, new strategies such as additional neuroprotection using hypothermia need to be explored, first to bridge time to reperfusion and second, to attenuate reperfusion injury.
Although therapeutic hypothermia has consistently demonstrated robust neuroprotection in animal ischemic-reperfusion models, randomized trials in acute stroke patients have failed to demonstrate the efficacy of induced hypothermia. The reasons for this treatment failure are diverse and include treatment delay, the unfeasibility of inducing and maintaining hypothermia due to intolerance and shivering in awake patients, the missing recanalization in a large proportion of patients, the heterogeneity of patients included, and too deep (32-34°C) hypothermia associated with an increase in side effects.
In the pilot study COTTIS-1, we could demonstrate the feasibility and safety of immediately induced intraischemic hypothermia to 35°C with non-invasive transnasal cooling by RhinoChill® (BrainCool) followed by surface cooling by BrainCool system® (BrainCool) for 6h after recanalization in sedated and intubated patients with LVO undergoing EVT. By combining this cooling technique with thrombectomy we have tried to address the above mentioned reasons for hypothermia failure. In COTTIS-1, the target temperature of 35°C was reached within 30 min, corresponding to a cooling rate of 2.6°C/h. All patients reached the target temperature, and 86% of the patients had reached ≤35°C at recanalization by thrombectomy. 68% of patients had a good outcome (independency) after 3 months. There were only asymptomatic side effects during hypothermia.

As a consequence, the present COTTIS-2 study is planned to evaluate the efficiency of this cooling protocol in a multicentric, randomized, controlled, end-point-blinded study in Germany.
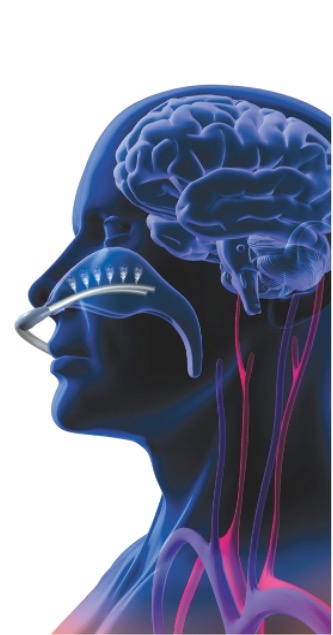
Three thermal components are used by RhinoChill
- The conduction (thermal cold conduction through direct contact) between the nasal catheter with its open end pieces and the mucous membrane of the nasopharynx via the thin, bony structure of the lamina cribrosa results in the transfer of the thermal cooling energy to the base of the skull.
- The readily soluble PFC coolant evaporates in the nasopharynx and achieves the cooling effect for brain protection (evaporation).
- The cooling energy is absorbed by the brain, released to the blood and circulated (thermal flow through moving medium = convection). The advantage is that the brain primarily absorbs this protective cooling effect and hypothermia develops only later through the blood circulation in the entire body.